Retatrutide vs Mounjaro (UK): the simple, evidence‑based guide
Quick safety note (UK): retatrutide is still investigational, and the MHRA has already seized unlicensed retatrutide and tirzepatide pens from an illegal UK facility — so it's best not to treat "random online supply" like it's the same as a pharmacy.
If you're on Mounjaro (tirzepatide) and you've started hearing people talk about retatrutide, you're not alone. Retatrutide has had some seriously attention‑grabbing trial results — but it's also not approved yet, which changes what's possible (and what's safe).
This article is for normal humans, not scientists. I'll explain what retatrutide is, how it compares to Mounjaro, what the side effects look like, why the "grey market" is such a big deal, and how to track your progress properly inside Healthcount.
Quick facts (30 seconds)
| Question | Quick answer |
|---|---|
| Is retatrutide approved in the UK? | Not yet. It's still being tested in Phase 3 trials. |
| What is retatrutide? | A once‑weekly injection that targets three hormone receptors (a "triple agonist"). |
| What is Mounjaro? | A once‑weekly injection that targets two hormone receptors (a "dual agonist"). |
| Can you convert Mounjaro mg → retatrutide mg? | No official conversion exists. You can only compare trial averages (roughly), not "equivalent doses". |
| What's the biggest risk right now? | Unregulated/illegal supply. The MHRA has warned and seized unlicensed pens. |
If you're trying to make a switch (or even just thinking about it), the most helpful thing you can do today is keep clean records
Healthcount is built for food, exercise, measurements, progress photos, and Mounjaro dosing logs.
Track Your ProgressWhat retatrutide is
Retatrutide is an investigational (not‑approved‑yet) medicine being tested for obesity and related conditions.
In a published Phase 2 trial (48 weeks), adults with obesity who took retatrutide lost, on average, more weight at higher doses, with the 12 mg group showing −24.2% average weight change at 48 weeks (compared with −2.1% in the placebo group).
It's usually described as a triple agonist because it activates three receptors:
- GLP‑1 receptor
- GIP receptor
- Glucagon receptor

What Mounjaro is
Mounjaro is the brand name for tirzepatide. In the UK, it has an official SmPC (Summary of Product Characteristics) that explains how it should be prescribed and used.
Mounjaro is a dual agonist, meaning it activates two receptors:
- GLP‑1 receptor
- GIP receptor
The UK SmPC also lays out how dosing usually works (start low and increase slowly): starting dose 2.5 mg once weekly, then move to 5 mg, then step up in 2.5 mg increments if needed (with at least 4 weeks at each step).

Tiny biology lesson: receptors and hormones
You don't need a biology degree for this — you just need the right mental picture.
What's a receptor?
A receptor is like a doorbell on a cell. When the right "finger" (a hormone or a medicine) presses it, the cell gets a message and reacts.
What's a hormone?
A hormone is a message chemical your body makes. Many hormones travel in your bloodstream (so yes, they're "in your blood"), but they aren't a separate liquid — they're molecules carried by the blood.
Why does this matter for weight and blood sugar?
Because drugs like Mounjaro and retatrutide are basically messages you inject, designed to press certain "doorbells" that affect:
- hunger and fullness
- how your body handles sugar after meals
- sometimes energy use
If you've ever wondered why these meds can change appetite so strongly… that's why.
Retatrutide vs Mounjaro: the big difference
Here's the headline:
- Mounjaro = dual agonist (2 targets)
- Retatrutide = triple agonist (3 targets)
So retatrutide has an "extra lever" (the glucagon receptor) on top of the GLP‑1 and GIP effects.
Important: you can't compare them by "milligrams" alone
This is the part that saves you from a lot of confusion:
- 1 mg of Drug A is not automatically "stronger" or "weaker" than 1 mg of Drug B
- They're different molecules, hitting different receptors, with different potencies and different dose–response curves
- So there is no official Mounjaro‑to‑retatrutide conversion chart
- What you can do is compare trial averages at specific timepoints — with a big "rough comparison" label
"What dose of retatrutide equals my Mounjaro dose?"
Let's answer this the way your readers are actually asking it.
The honest answer (the one you can trust)
There is no evidence‑based, safe, "same impact" conversion from Mounjaro mg to retatrutide mg — especially because retatrutide isn't approved yet.
What we can do is compare published trial results at different doses and see what looks similar on average (not person‑to‑person).
A clear, rough comparison table (trial averages, not a dosing guide)
Not a conversion chart. This is only to help you understand why "mg" doesn't translate cleanly. Different studies, different timeframes, different people.
| Medicine | Dose group | Timepoint | Average weight change |
|---|---|---|---|
| Mounjaro (tirzepatide) | 5 mg weekly | 72 weeks | −16.0% |
| Mounjaro (tirzepatide) | 10 mg weekly | 72 weeks | −21.4% |
| Mounjaro (tirzepatide) | 15 mg weekly | 72 weeks | −22.5% |
| Retatrutide (investigational) | 1 mg weekly | 48 weeks | −8.7% |
| Retatrutide (investigational) | 4 mg weekly (combined 4 mg groups) | 48 weeks | −17.1% |
| Retatrutide (investigational) | 8 mg weekly (combined 8 mg groups) | 48 weeks | −22.8% |
| Retatrutide (investigational) | 12 mg weekly | 48 weeks | −24.2% |
Let's explain:
- In trials, retatrutide 4 mg at 48 weeks landed around the same average weight‑loss range as Mounjaro 5 mg at 72 weeks — but that does not make them equivalent doses.
- And for higher doses: retatrutide 8–12 mg shows higher average loss at 48 weeks, while Mounjaro 10–15 mg shows strong results at 72 weeks.
Will you have to "dose ladder" again if you switch from Mounjaro to Retatrutide?
Very likely, yes.
Why? Because the slow step‑up is how these medicines reduce side effects, especially nausea and vomiting.
- Mounjaro's UK SmPC describes step‑ups in 2.5 mg increments after at least 4 weeks at a dose.
- In the Phase 2 retatrutide trial, some groups used a lower starting dose (2 mg vs 4 mg) and the study notes side effects were "partially mitigated" with a lower starting dose.
To be on the safe side: Even if you've used Mounjaro before, a new medicine will usually mean a fresh titration schedule (if and when it's approved), because tolerability matters.
What retatrutide is being studied for
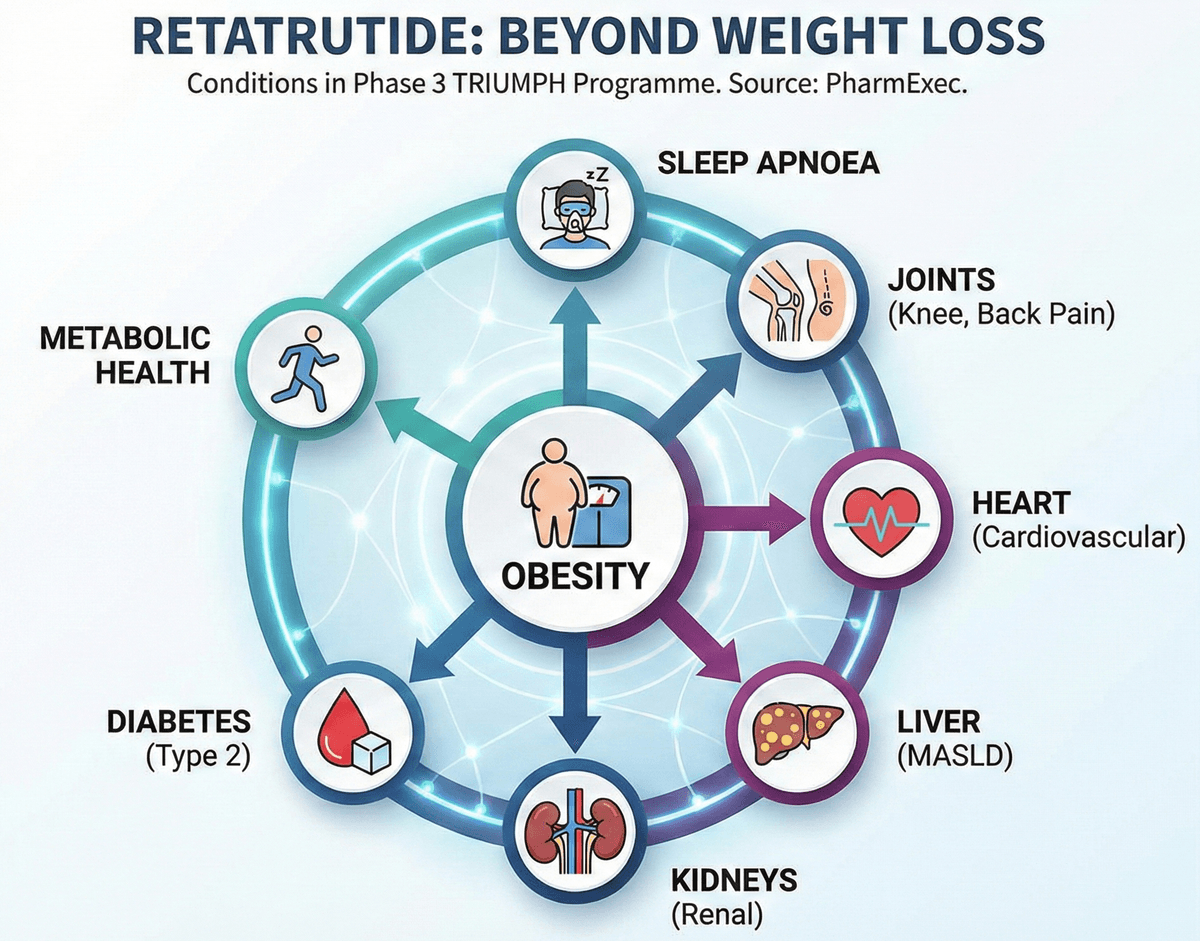
Retatrutide isn't just being studied for "weight loss". The Phase 3 TRIUMPH programme includes multiple conditions linked to obesity (and the complications that come with it).
Here's the clean list that's been publicly described:
| Category | Examples in the Phase 3 programme |
|---|---|
| Weight management | Obesity, overweight with comorbidities |
| Metabolic health | Type 2 diabetes |
| Pain and mobility | Knee osteoarthritis; chronic low back pain |
| Sleep / breathing | Obstructive sleep apnoea |
| Heart / kidneys | Cardiovascular outcomes; renal outcomes |
| Liver | Metabolic dysfunction‑associated steatotic liver disease (MASLD) |
Current status in the UK and what might happen next
Is retatrutide available in the UK right now?
Not as a normal prescription medicine.
As of December 2025, retatrutide is still an investigational medicine in Phase 3 trials.
What's new recently?
Lilly's first Phase 3 readout (TRIUMPH‑4) reported strong results in people with obesity/overweight and knee osteoarthritis, including:
- ~28.7% average weight loss at 68 weeks at the 12 mg dose (topline reporting), plus improvements in pain and function measures
The important phrase there is "topline". It's encouraging, but the full peer‑reviewed dataset is what eventually sets the standard.
What might happen next year?
More Phase 3 readouts are expected in 2026 (again, that's based on public reporting around the trial programme).
Key Takeaway: Retatrutide is moving quickly through trials, but approval timing is still uncertain until regulators review the full evidence.
Side effects: what's common (and what's worth watching)
Most of the side effects people talk about for these meds are… honestly… stomach‑related. That's not because the drug is "bad", it's because these hormone pathways affect appetite and digestion.
Common side effects seen in retatrutide reporting
In the Phase 2 trial, the most common adverse events were gastrointestinal and dose‑related, mostly mild to moderate.
In TRIUMPH‑4 reporting, GI effects like nausea, diarrhoea, constipation, vomiting, and decreased appetite were also common.
One side effect that gets people asking questions: dysesthesia
TRIUMPH‑4 reporting mentions dysesthesia (odd, uncomfortable sensations like tingling/burning) and says it was generally mild and infrequently led to discontinuation.
| Type | Examples | What most people do with this info |
|---|---|---|
| GI (most common) | nausea, diarrhoea, constipation, vomiting, lower appetite | Expect it to be most noticeable during dose increases; talk to your prescriber if it's persistent. |
| Sensory / nerve‑type | dysesthesia (tingling/burning sensations) | Don't ignore new or worrying symptoms; get medical advice. |
| General | feeling more full, eating less (often "positive" but can be too much) | Track what's happening so you can spot patterns and keep nutrition sensible. |
The grey market: what it is and why it matters here
What does "grey market" mean?
In this context, it usually means products sold outside regulated pharmacies — often labelled "for research only".
Why it's especially relevant for retatrutide
Because retatrutide isn't approved yet, any "retatrutide for sale" to the general public is not coming through normal, regulated medicine supply chains.
And in the UK, this risk is not theoretical:
The MHRA announced it dismantled an illicit facility and seized more than 2,000 unlicensed retatrutide and tirzepatide pens, plus raw ingredients and equipment, and warned people not to buy weight‑loss meds from unregulated sources.
The simple truth
If it isn't coming from a legitimate clinic/pharmacy route, you cannot reliably know:
- what's in it,
- how strong it is,
- whether it was stored correctly,
- whether it's sterile.
That's a big deal for anything you inject.
What to track in Healthcount (so this doesn't become guesswork)
If you only do one thing after reading this, do this: track the basics consistently.
Not because you need to "obsess". Because your memory lies to you. (Mine does too.)
Here's a simple routine that fits real life.
The Healthcount tracking plan (simple and sustainable)
| What to track in Healthcount | Best frequency | Why it helps |
|---|---|---|
| Weight | Daily → weekly (weigh daily if you can, then look at your weekly average) | Daily weight jumps around (water, salt, sleep). Weekly averages show the real trend. |
| Measurements (waist, hips, etc.) | Monthly | Tape measures change slower than the scale, but they're brilliant for long‑term progress. |
| Progress photos | Weekly → monthly | Weekly if you like detail; monthly if you prefer less pressure. Consistency beats perfection. |
| Food | Daily (rough is fine) | Helps you spot patterns (weekends, snacks, drinks) without turning food into maths homework. |
| Exercise | Each workout | Lets you connect progress to behaviour changes ("I actually moved more this month"). |
| Dosing | Every dose | If something changes, you can line it up with the timeline. Learn about weekly injection timing. |

If you're already putting the effort in, you might as well get the benefit
Use Healthcount to build a clear "week by week" story you can actually trust.
Start TrackingFAQs
Can I switch to retatrutide right now in the UK?
Not through normal prescribing routes. Retatrutide is still investigational, so the clean path is clinical trials (or waiting for approval).
Is retatrutide "better" than Mounjaro?
It may end up being more effective for some people, but we're still waiting for more Phase 3 results and real‑world safety data. The early numbers are impressive, but "better" depends on tolerability, safety, and what you personally need.
If I'm doing well on Mounjaro, should I chase what's next?
Not automatically. If you're losing weight, feeling okay, and your health markers are improving, that's already a win. Newer isn't always better for you — sometimes it's just newer.
Do I have to titrate again if I switch medicines?
Very likely, yes. Both Mounjaro's prescribing info and retatrutide trial design reflect the idea of starting lower and stepping up to reduce side effects. For accurate dosing, check our conversion guide.
Sources
Retatrutide Phase 2 obesity trial
PubMed summary of the NEJM trial (includes dosing groups and weight‑loss results)
Mounjaro UK prescribing document (official SmPC)
MHRA warning on unlicensed pens
Phase 3 TRIUMPH programme
Written by Anna Bromley, Healthcount Founder
Last reviewed: 13 Dec 2025